

The answer is rounded to two sig figs, the number of sig figs you have for your values. Magnesium chloride is made out of one magnesium atom and two chlorine atoms. To convert this to grams, use the compound's molar mass So, you know that you can get a #"4.5-M"# magnesium chloride solution by dissolving #9.0# moles of magnesium chloride in enough water to have a total volume of #"2.0 L"# of solution. Magnesium chloride (MgCl2) Cl2Mg CID 24584 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological. atomic mass of oxygen2.atomic mass of chlorine3. chloride and magnesium oxide appears to slightly decrease low-density. Calculate the mass of oxygen consumed during the reaction and the mass of magnesium oxide produce. Magnesium - Atomic Mass - Atomic Weight - Mg. Magnesium Chloride molecular weight Molar mass of MgCl2 95.211 g/mol Convert grams Magnesium Chloride to moles or moles Magnesium Chloride to grams Molecular weight calculation: 24.3050 + 35.4532 Percent composition by element Element: Magnesium Symbol: Mg Atomic Mass: 24.3050 of Atoms: 1 Mass Percent: 25. Atomic mass of Magnesium (Mg) 24.305: 24: 13: Atomic mass of. 12.2 g of magnesium metal reacts completely with oxygen gas to produce magnesium oxide. #(color(red)(?)color(white)(.)"moles MgCl"_2)/"2.0 L solution" = overbrace("4.5 moles MgCl"_2/("1.0 L solution"))^(color(blue)("= 4.5 M solution"))# Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na +.
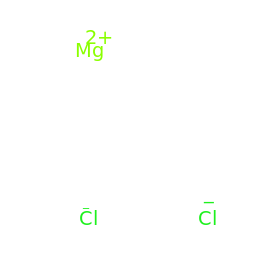
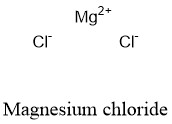
So all you have to do now is figure out how many moles of magnesium chloride would be equivalent in #"2.0 L"# of solution to #4.5# moles in #'1.0 L"# of solution. The same concept can be extended to ionic compounds and molecules. This implies that a #"4.5-M"# magnesium chloride solution will contain #4.5# moles of magnesium chloride, the solute, for every #"1.0 L"# of solution. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. For starters, we know that a #"1-M"# solution contains #1# mole of solute for every #"1.0 L"# of solution. Element Magnesium (Mg), Group 2, Atomic Number 12, s-block, Mass 24.305.


 0 kommentar(er)
0 kommentar(er)
